NOVOCART 3D is an investigational product and the subject of a Phase 3 clinical trial in the United States.
It has been used since 2003 in a select few European countries. It is considered an autologous chondrocyte implantation product, meaning that the patient’s own cartilage is harvested and cultivated to produce the implant. The cultivated cells are distributed on a biphasic three dimensional collagen scaffold (like a sponge). The scaffold and cells may be implanted into the articular cartilage of the knee, once the damaged cartilage is removed.
The procedure takes place over the course of two surgeries:
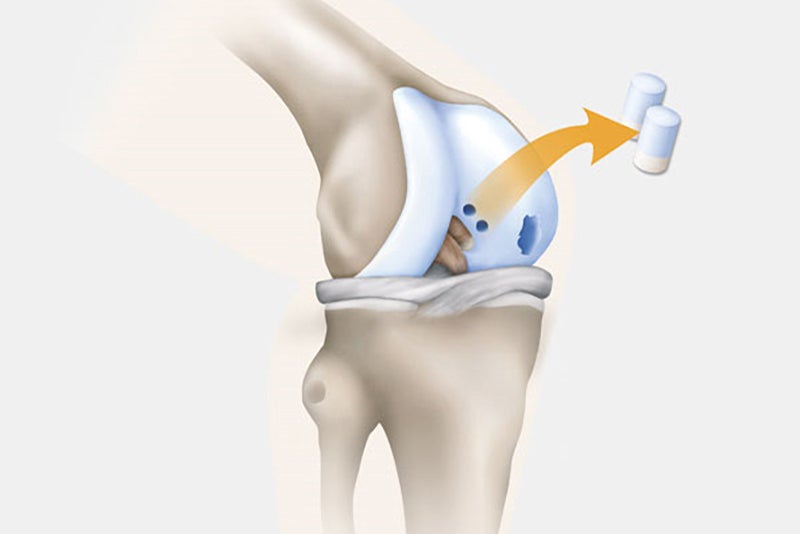
Step 1: Cartilage Extraction
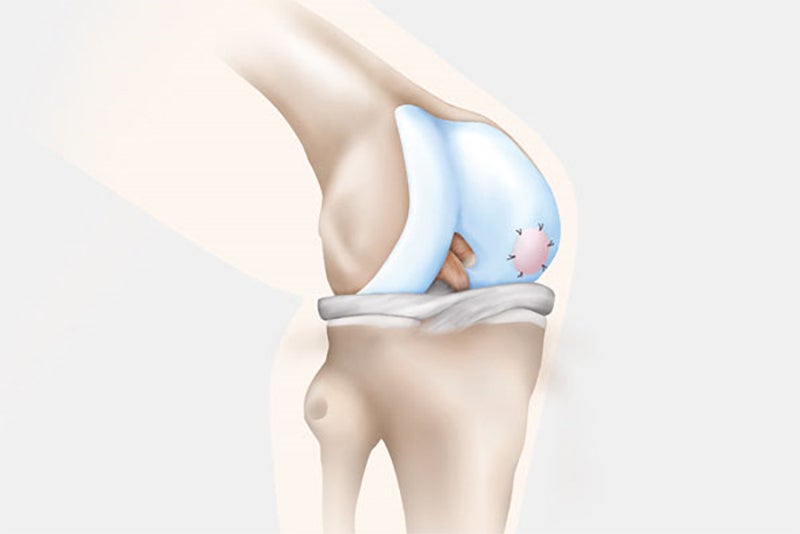
Samples of the patient’s knee cartilage are removed from a non-weight bearing location in the knee joint. The surgery can be done on an outpatient basis via an arthroscopy.
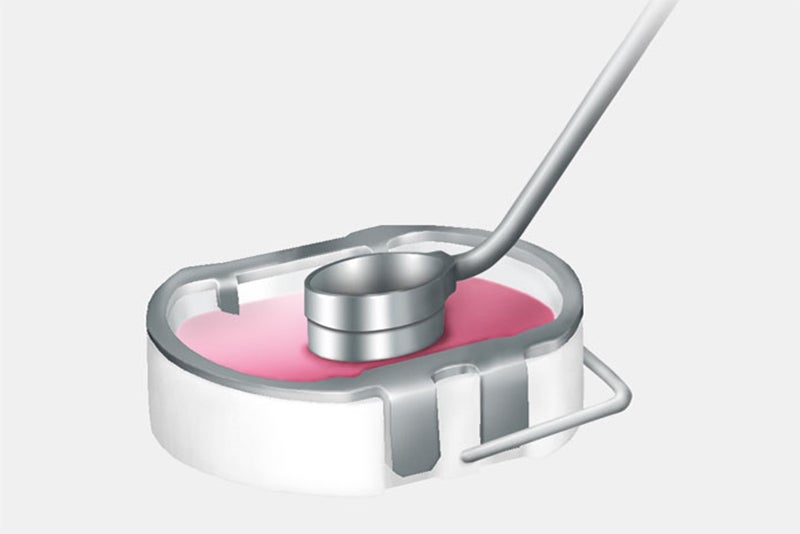
The cartilage samples are sent to the Aesculap Biologics manufacturing laboratory where the cells (chondrocytes) are separated from the surrounding cartilage structure and cultivated. Approximately three weeks later, the cells are added to a bilayer collagen sponge, which to a great extent matches the original biological cell environment, where they distribute themselves and attach to the scaffold fibers. This cell/sponge combination is called NOVOCART 3D.
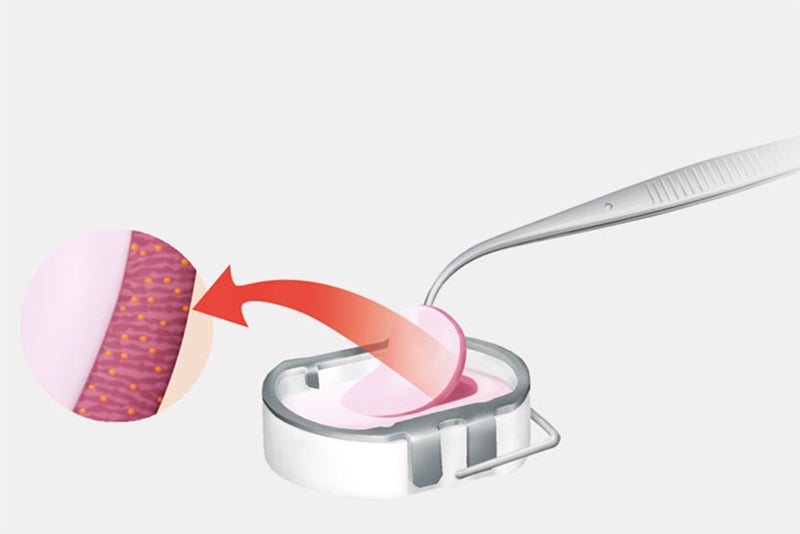

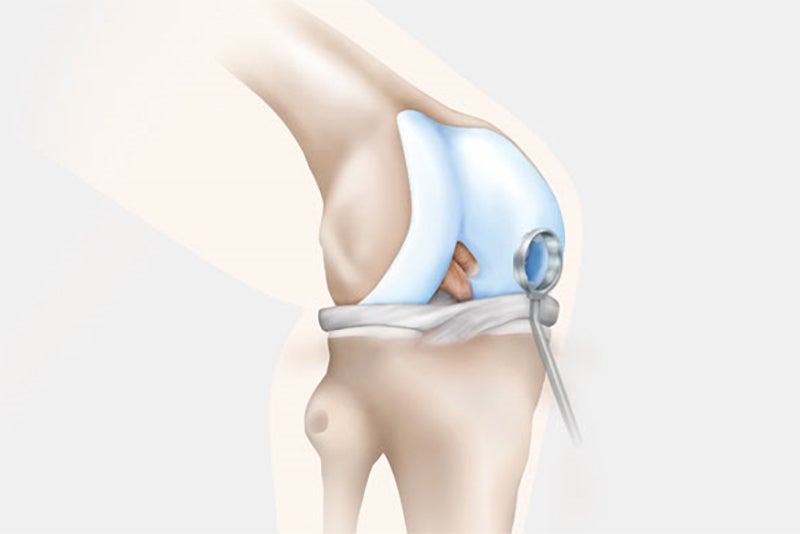
Step 2: Transplantation
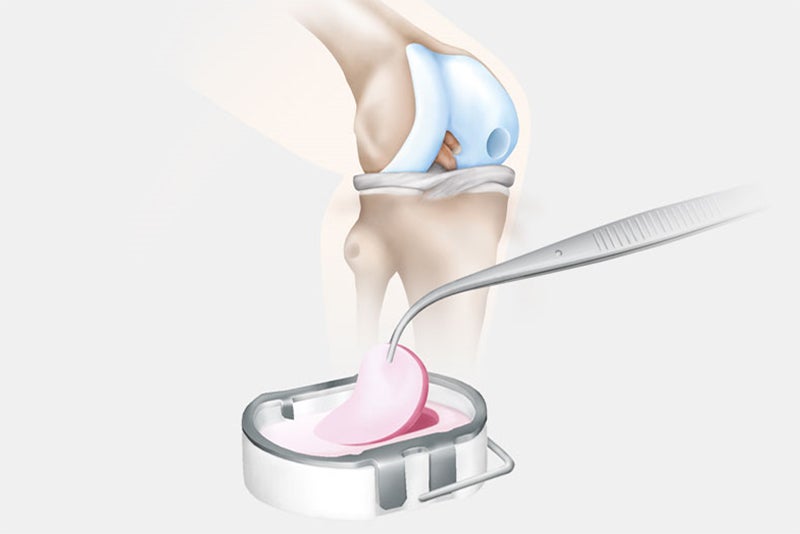
In the second surgery, the orthopaedic surgeon accesses the joint and removes the damaged cartilage through a small incision called an arthrotomy. NOVOCART 3D is shaped to fit the space that remains when the damaged cartilage is removed and NOVOCART 3D is implanted. The implant is fixed with absorbable sutures and/or biodegradable pins and the operation wound is closed. In some studies where cartilage has been sampled after implantation of NOVOCART 3D, normal cartilage has been detected.
All surgical interventions have associated risks. Please consult with your doctor to discuss risks and benefits.